The indicators under development are based on a chemical reaction between permanganate and oxalate.
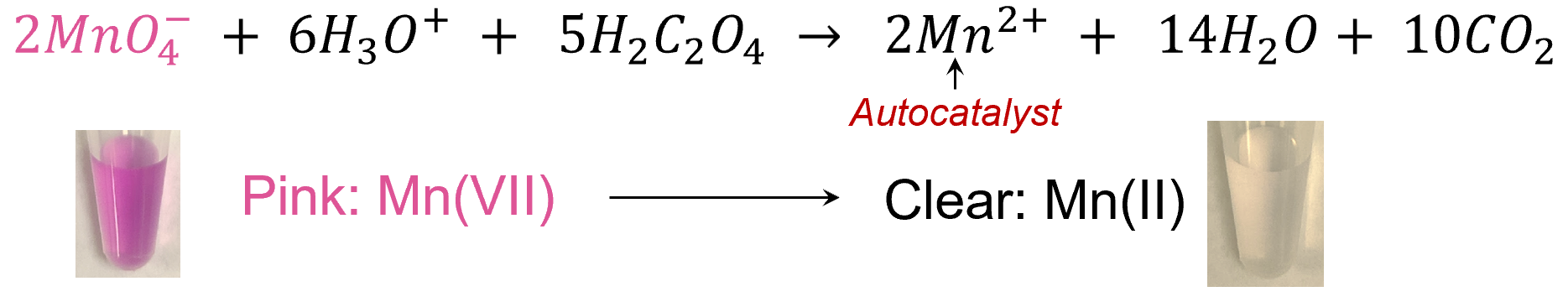
This reaction has unique, mathematically modelable autocatalytic kinetics, facilitating the design of custom run times (at a fixed temperature) during which the color remains a vivid pink color then rapidly transitions to clear at the end of the designed run time:
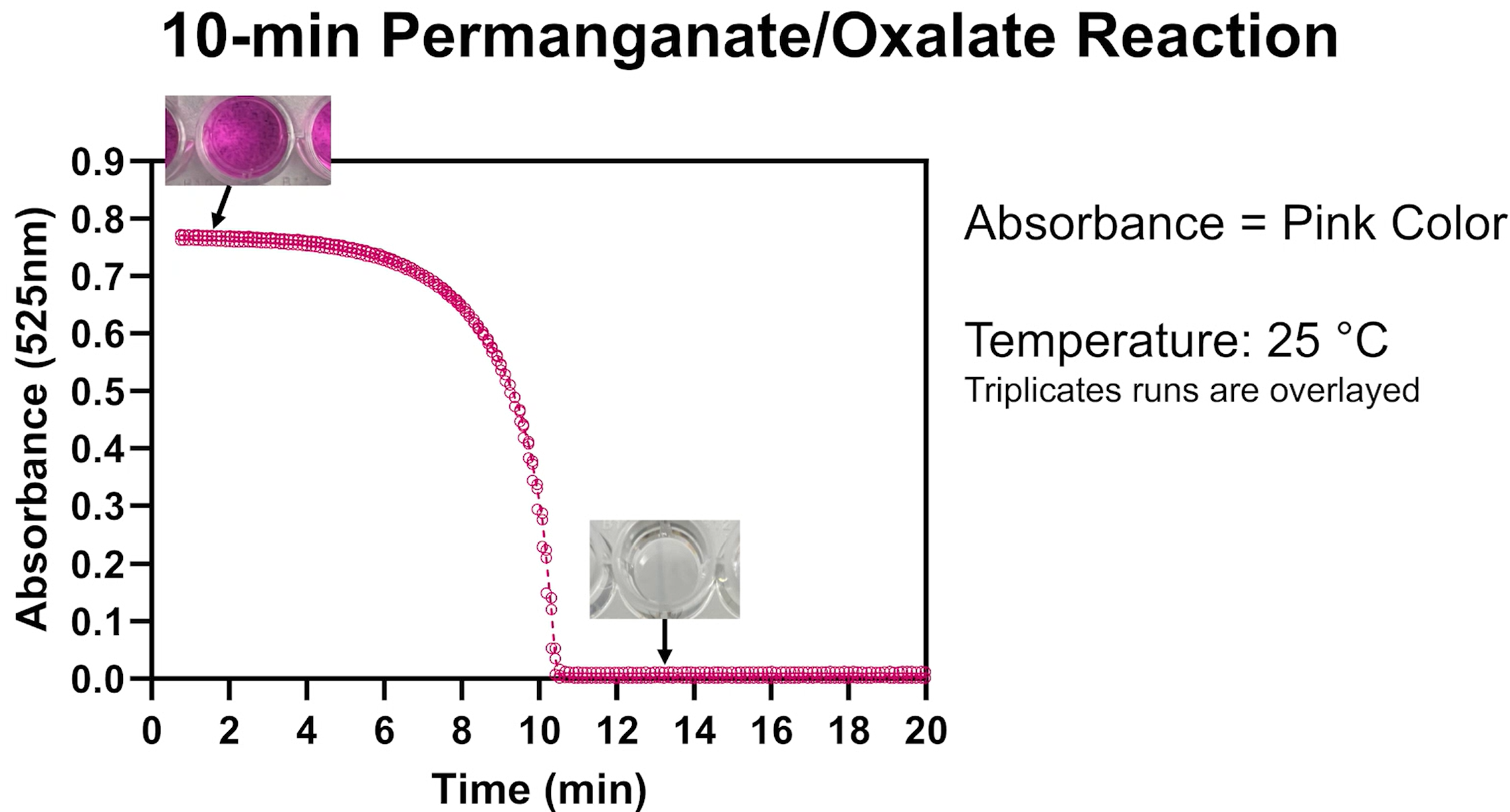
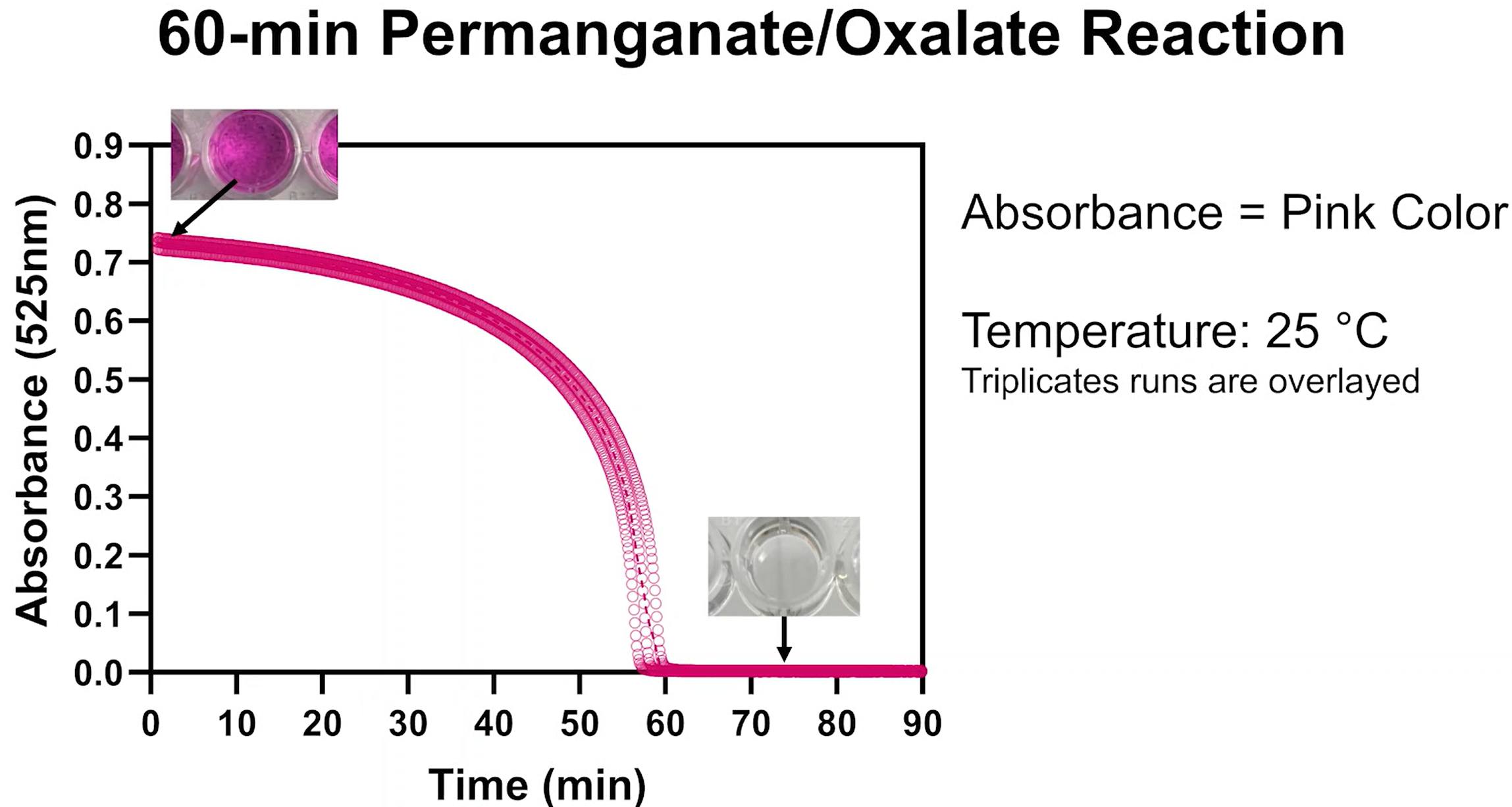


If the temperature changes while the reaction is running, the reaction speeds up (at warmer temperatures) or slows down (at colder temperatures), mirroring the kinetics of reactions that lead to biomolecular degradation:
In the time-lapse video below a single reaction solution is split and run at room temperature and on ice. The room temperature indicator expires after 12 minutes. The indicator on ice takes 60 minutes to expire:
The reactions can be run in special anti-freeze solutions that allow for the indicators to remain active well below 0 °C, but eutectically freeze (and therefore halt) at temperatures below -17 °C, -37 °C, or -67 °C. The graphs below illustrate the ability of these indicators to continue to run well below 0 °C. They also illustrate how the reactions speed up dramatically with increased temperature—as desired for a visual indicator that tracks biomolecular integrity:
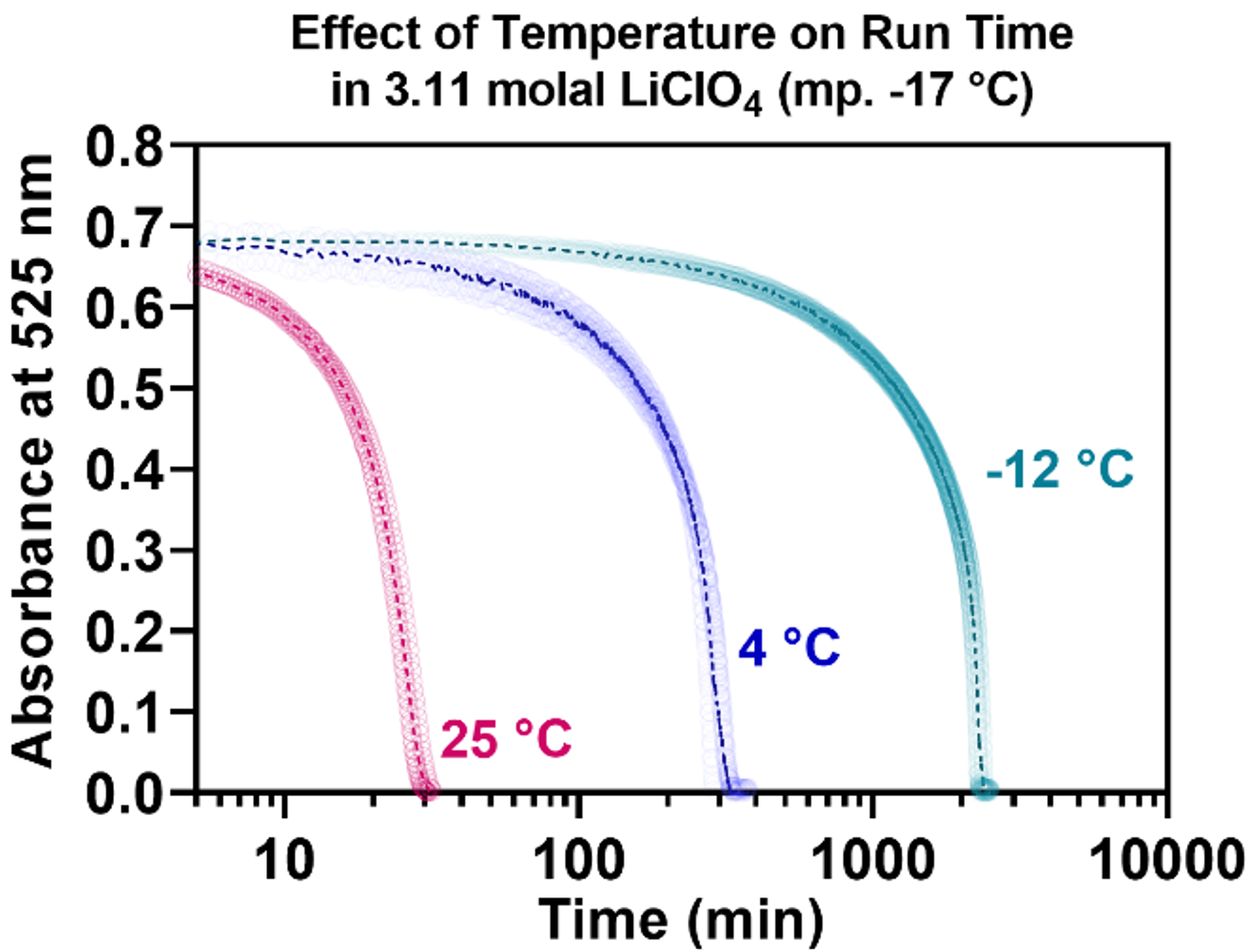
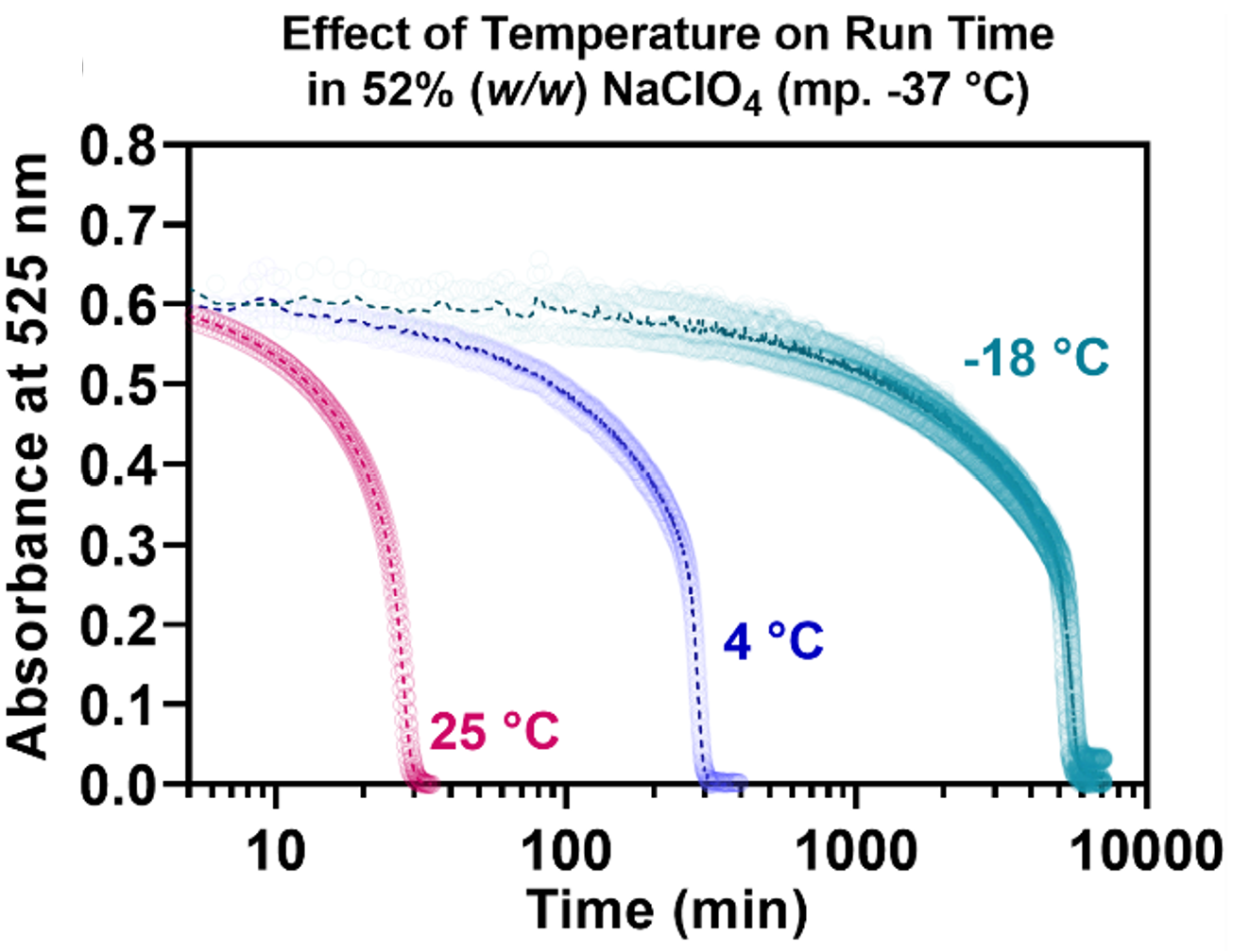
2runsat25_4_-18c.png)


2runsat25_4_-18c.png)